Blogs

Is Iron a Magnetic Element? Your Essential Checklist for Understanding Its Properties
Introduction
The magnetic properties of iron are not only fascinating from a scientific perspective but also critical for a wide array of industrial applications. Understanding ferromagnetism—the phenomenon that allows iron to become magnetized—unlocks insights into its unique characteristics, such as robust magnetic behavior and the ability to retain magnetization.
This article delves deep into the fundamental principles of iron’s magnetism, exploring:
- Its atomic structure
- The role of unpaired electrons
- The influence of temperature and external factors on its magnetic properties
Furthermore, it compares iron with other ferromagnetic metals like nickel and cobalt, highlighting their distinct advantages and limitations.
By examining the practical applications of iron’s magnetism in:
- Electrical appliances
- Data storage
- Emerging technologies
Procurement managers can make informed decisions that align with project requirements and safety standards, particularly in environments where non-sparking tools are essential. This comprehensive exploration will equip professionals with the knowledge needed to navigate the complexities of material selection in today’s dynamic industrial landscape.
Understanding Iron’s Magnetic Properties: The Basics of Ferromagnetism
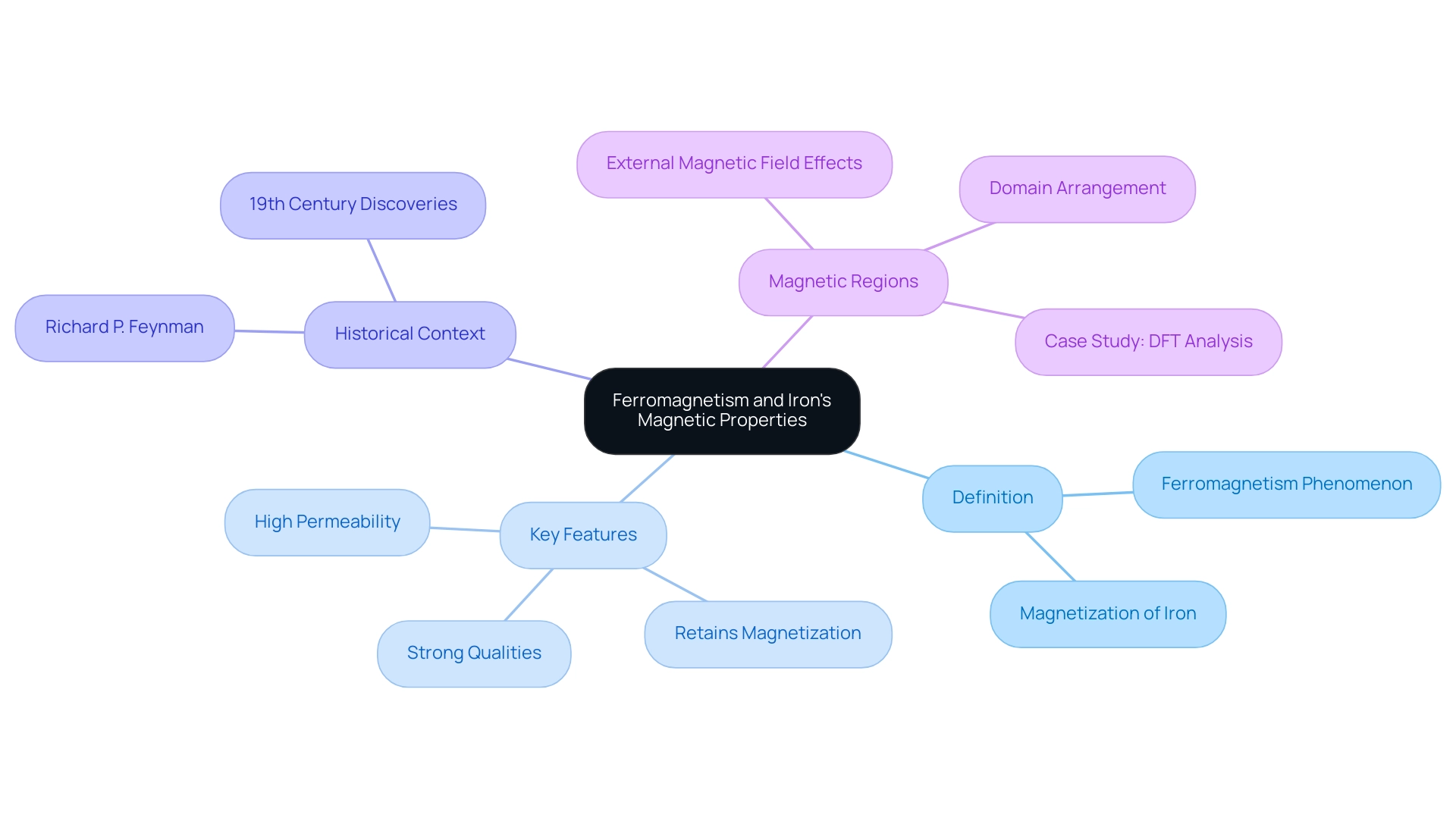
Define Ferromagnetism: Ferromagnetism refers to the phenomenon in which certain substances, particularly in the context of whether is iron a magnetic element, can be magnetized due to the parallel alignment of their atomic moments. This inherent characteristic allows these substances to display powerful fields, rendering them crucial in numerous applications, ranging from electrical engineering to data storage.
Identify Key Features: Ferromagnetic materials are characterized by several essential features:
- They possess strong qualities.
- They can retain their magnetization after the removal of an external field.
- They exhibit high permeability, allowing them to effectively channel lines of force.
The stability of their remanent magnetization is particularly noted in recent studies, which indicate that upon both compression and decompression, the remanent magnetization can increase significantly, as highlighted by Stuart A. Gilder, who noted that ‘the remanent magnetization of the body centered cubic phase increases several times over initial conditions while the coercivity of remanence remains mostly invariant with pressure.’ Additionally, statistics from García et al. (2022) further support these claims, demonstrating the remarkable properties of ferrous material under various conditions.
Explore Historical Context: The examination of magnetism possesses a rich historical background, especially with a certain metal being acknowledged as a ferromagnetic substance in the 19th century. Innovative contributions, like those from Richard P. Feynman in ‘The Feynman Lectures on Physics,’ established essential knowledge that still impacts contemporary comprehension and uses of substances today.
Understand Magnetic Regions: The ferrous behavior is largely governed by the arrangement of its domains. These domains can be thought of as small, magnetically polarized regions within the material. When exposed to an external magnetic field, these domains align, thereby enhancing the overall magnetism of the material. Comprehending whether is iron a magnetic element is essential for uses that depend on the attributes of iron, especially regarding new studies on different iron compounds and their applications. For instance, the case study titled ‘DFT analysis of supra-molecular assemblies of substituted 4H-pyran derivatives’ illustrates how Density Functional Theory (DFT) can be employed to analyze the structural and electronic characteristics of compounds that may exhibit ferromagnetic properties.
Key Factors Influencing Iron’s Magnetism: Atomic Structure and Electron Configuration
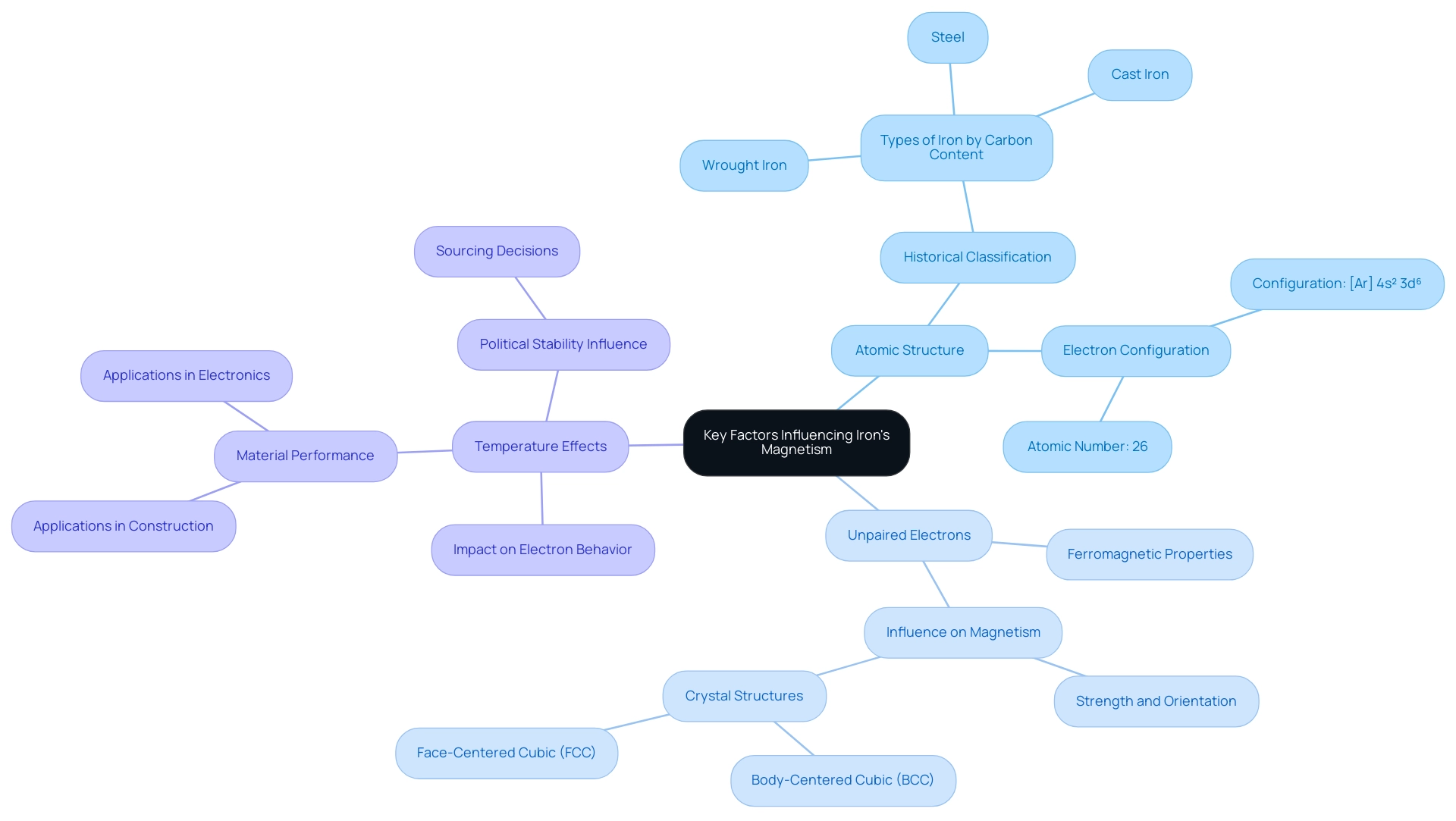
Analyze Atomic Structure: Iron, with an atomic number of 26, possesses an electron configuration of [Ar] 4s² 3d⁶. This configuration reveals the arrangement of electrons across its energy levels, which is foundational to understanding its magnetic characteristics. Given that the global stock of this metal in use is approximately 2,200 kg per capita, comprehending its atomic structure is not only academically significant but also pivotal for various industrial applications. Historically, Rene Antoine Ferchault de Reaumur categorized types of metal in 1722, distinguishing between steel, wrought metal, and cast metal based on carbon content, which is essential for understanding the substance’s evolution and applications.
Focus on Unpaired Electrons: A key factor in the properties of this element lies within its unpaired electrons located in the 3d orbital. These unpaired electrons are crucial in producing the moment of this element, classifying it as ferromagnetic. The comprehension of these unpaired electrons is essential, as they directly influence the strength and orientation of the magnetism in this metal, which raises the question of whether iron is a magnetic element, a crucial consideration in procurement and material selection. The crystal structure of the metal, particularly the body-centered cubic (BCC) and face-centered cubic (FCC) configurations, is iron a magnetic element that significantly affects its properties related to magnetism. Each structure presents varying attractions due to the arrangement of atoms and the resultant effects on the unpaired electrons, which dictate how the element interacts with external fields.
Consider Temperature Effects: Temperature plays a crucial role in modifying the behavior of electrons within the metal. Variations in temperature can lead to changes in electron distribution and magnetic properties, affecting the material’s performance in applications ranging from construction to electronics. Comprehending these temperature-related changes is crucial for procurement managers when assessing iron-based substances for specific environments. Furthermore, as mentioned by the British Geological Survey, the political stability of nations with substantial mineral reserves can influence procurement choices, highlighting the necessity for strategic sourcing. Additionally, the case study titled ‘Role of Metal in Oceans’ illustrates this element’s ecological significance, highlighting its critical role in ancient marine ecosystems and climate, which procurement managers should consider for sustainability in material choices.
Iron vs. Other Magnetic Metals: A Comparative Analysis
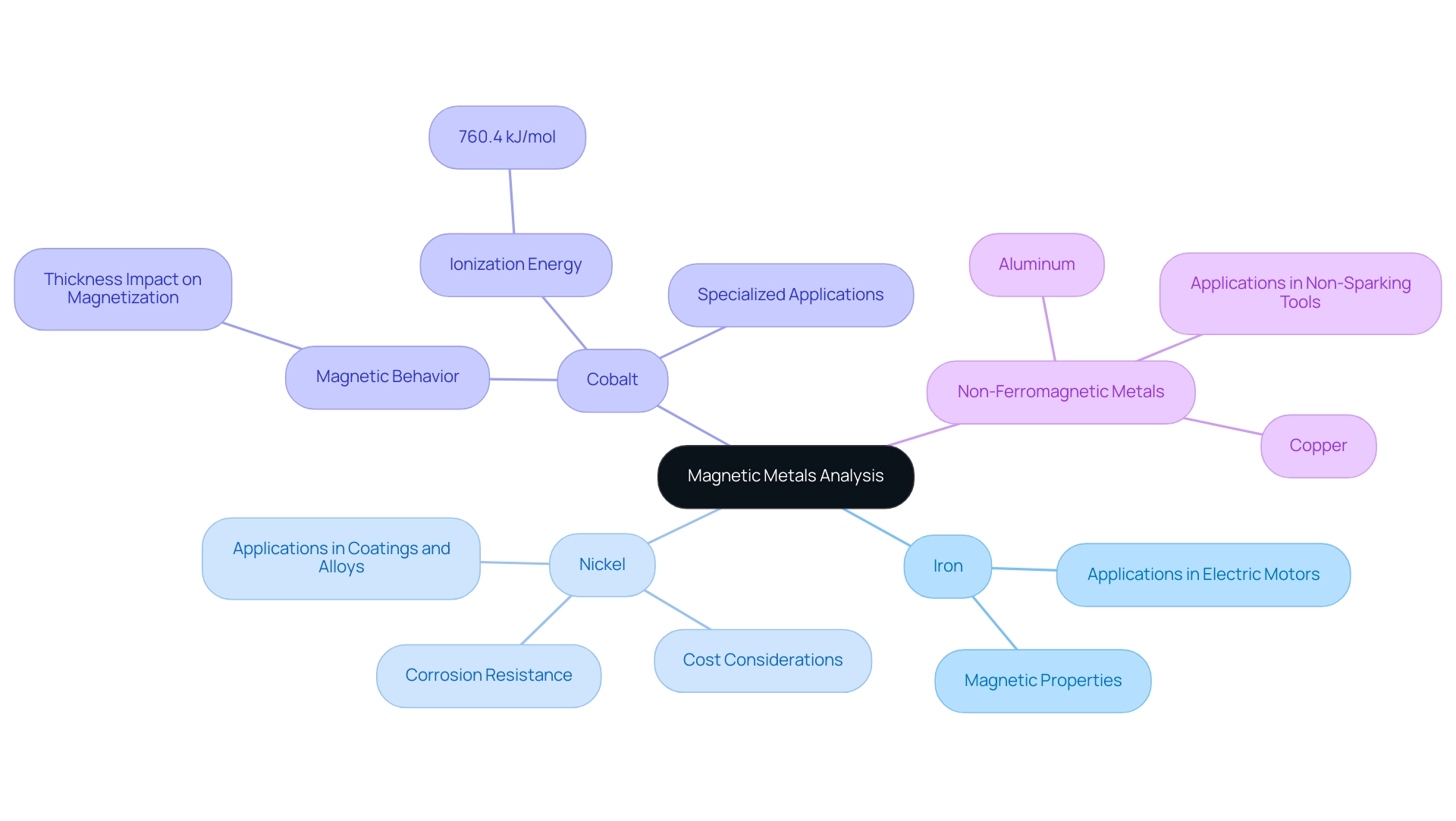
- Comparison with Nickel: Nickel shares ferromagnetic properties with a certain metal, making it suitable for various applications. However, its unique corrosion resistance and higher cost often limit its use in certain environments, particularly in explosive potential settings. While iron is commonly employed in electric motors and transformers, nickel is advantageous in applications requiring enhanced resistance to oxidation, such as coatings and alloys. This nuanced differentiation enables procurement managers to make informed decisions based on specific project requirements. Significantly, in settings where non-sparking tools are essential, the selection of substances becomes crucial to ensure safety and compliance. Suppliers like [Supplier Name] and [Supplier Name] specialize in providing high-quality non-sparking tools, which are vital for maintaining operational safety in explosive environments. Moreover, the distinctive soft characteristics of amorphous metal-metalloid alloys are noteworthy, as they can provide extra choices in material selection while considering non-sparking features.
Evaluation of Cobalt: Cobalt is another ferromagnetic metal, recognized for its strong electromagnetic properties. Despite its advantages, cobalt’s higher cost and relative scarcity compared to iron hinder its widespread application. Recent studies highlight cobalt’s magnetic behavior, revealing that its magnetization does not significantly decrease with reduced thickness, which positions it favorably in specialized applications. The case study titled ‘Magnetic Properties of Cobalt Plates Compared with Iron and Nickel Plates‘ supports this, indicating that cobalt plates exhibit a consistent factor of magnetization regardless of thickness. Yoshiaki Tsunawaki notes, ‘Furthermore, we estimated three types of demagnetizing factor depending on the thickness of the plate,’ underscoring the importance of thickness in cobalt’s performance. Additionally, cobalt’s ionization energy of 760.4 kJ/mol further highlights its distinctive characteristics. In explosive environments, the use of non-ferromagnetic and non-sparking materials is paramount to ensure safety.
Highlighting Non-Ferromagnetic Metals: Unlike iron, non-ferromagnetic metals such as aluminum and copper lack attraction to magnets, making them unsuitable for applications that require ferromagnetism. These metals are often chosen for their conductivity or lightweight characteristics but do not contribute to magnetic functionalities. Understanding the distinctions between ferromagnetic and non-ferromagnetic materials is crucial for procurement managers when selecting materials for specific applications, especially non-sparking tools in hazardous environments. Suppliers like [Supplier Name] provide non-sparking options that meet industry standards for safety.
Discussion of Applications: The distinctive characteristics of iron raise the question of whether iron is a magnetic element, which makes it the metal of choice for uses like electric motors, where efficient magnetization is essential. The effective performance of iron in various electromagnetic devices prompts the inquiry, is iron a magnetic element due to its soft attractive properties? Conversely, cobalt finds niche applications due to its superior attractive properties, though its higher expense often restricts its use to specialized fields. By understanding the specific advantages and limitations of each ferromagnetic metal, procurement managers can better align material choices with project needs and budget constraints, particularly in selecting non-sparking tools from reputable suppliers that ensure operational safety in explosive environments.
Applications of Iron’s Magnetism: From Electrical Appliances to Storage Devices
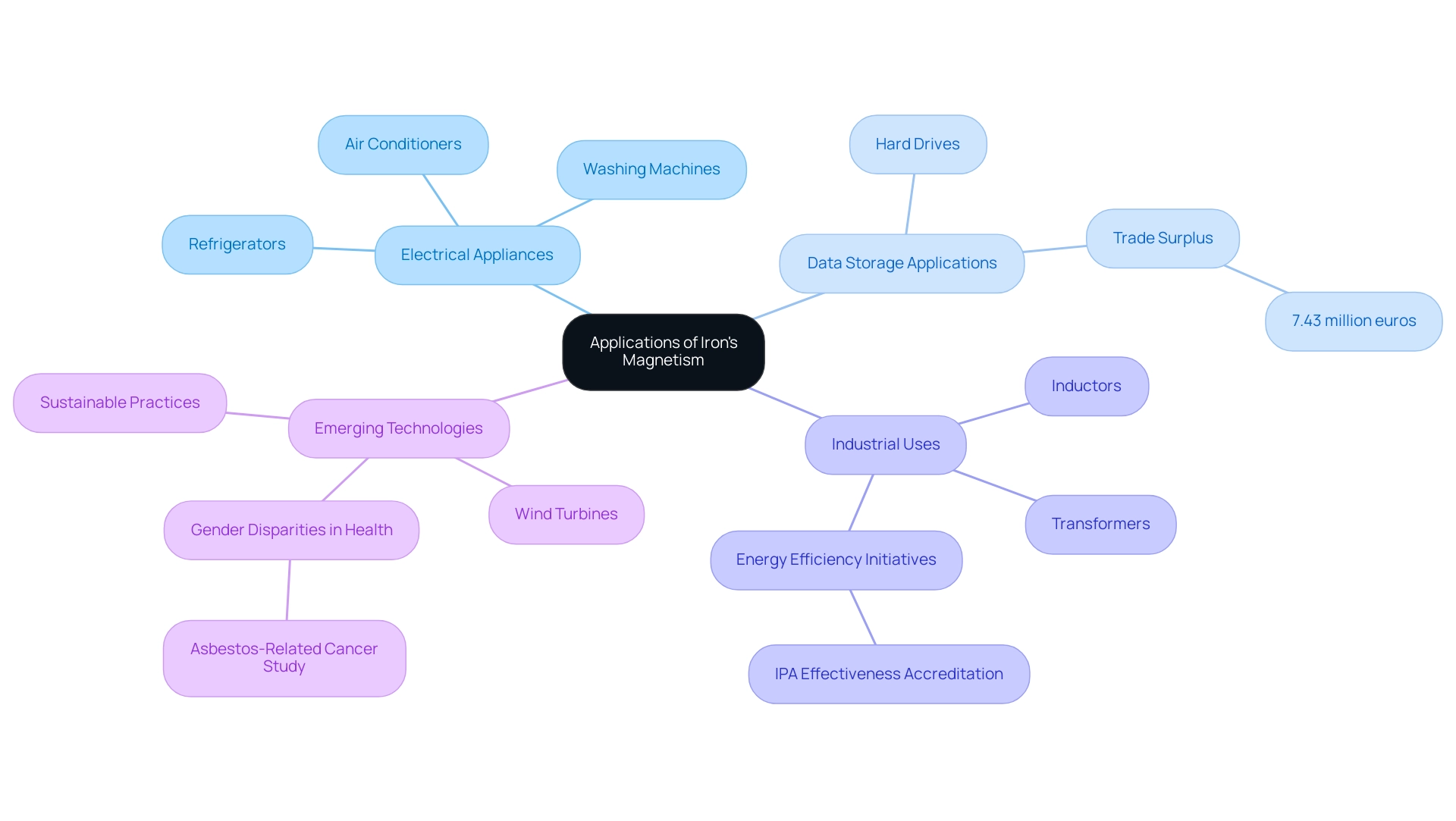
Identify Common Electrical Appliances: Recognize essential devices such as refrigerators, washing machines, and air conditioners that utilize the properties of metal in their motors. These components enhance functionality and efficiency, playing a pivotal role in daily operations.
Analyze Data Storage Applications: Investigate the essential role of metal in storage devices, particularly hard drives. The question of whether iron is a magnetic element highlights its unique attractive properties that facilitate robust data retention, making it integral to modern computing solutions. Notably, the global trade surplus in this sector was reported at 7.43 million euros in October 2023, highlighting the economic significance of these applications.
Highlight Industrial Uses: Examine the application of metal in transformers and inductors within electrical systems. Its attractive characteristics play a crucial role in energy efficiency, enhancing electrical flow and lowering operational expenses across different sectors. As Julian Douglas, the new IPA President, observed, initiatives such as the IPA Effectiveness Accreditation program are intended to improve the efficiency of resources, including metals, in various applications.
Explore Emerging Technologies: Assess the newest advancements in renewable energy technologies, like wind turbines, where the characteristics of metal are crucial. These advancements not only enhance energy production but also contribute to sustainable practices that address current environmental challenges. Furthermore, comprehending the differing effects of materials, as emphasized in the case study on gender disparities in asbestos-related cancer experiences, highlights the significance of considering various applications and consequences of substances in different contexts.
When Does Iron Lose Its Magnetism? Factors and Conditions
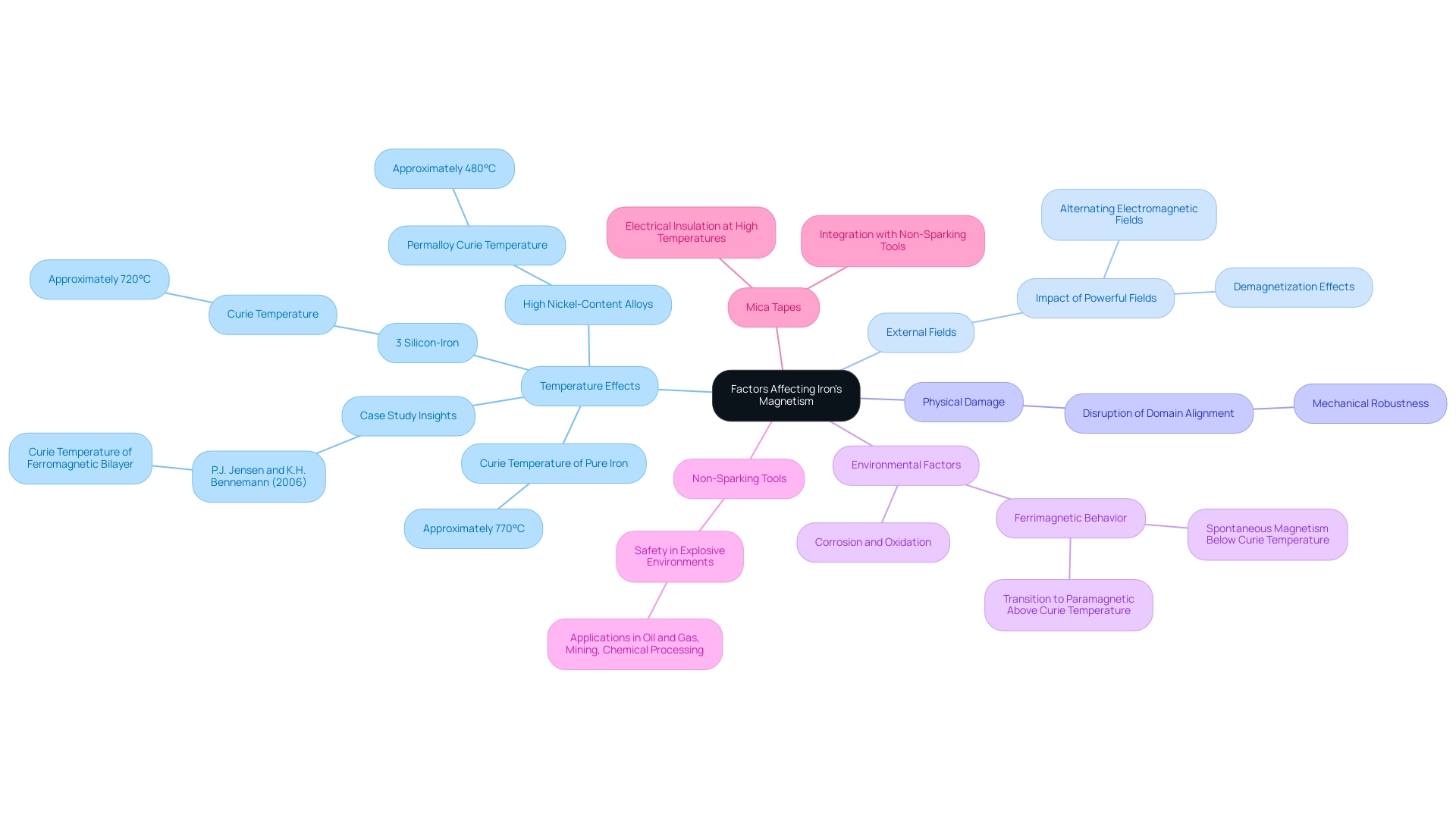
Identify Temperature Effects: Iron shows a significant alteration in its properties when exposed to heat. Specifically, it loses its magnetism when temperatures exceed its Curie temperature, which is approximately 770°C for pure iron, leading to the inquiry of whether is iron a magnetic element. This phenomenon is critical in applications where thermal conditions fluctuate. R.E. Hanitsch emphasizes that high nickel-content alloys, like Permalloy, possess a Curie temperature of approximately 480 °C, whereas 3% silicon–iron attains 720 °C, illustrating the variation in behavior based on alloy composition. Additionally, a case study by P.J. Jensen and K.H. Bennemann (2006) investigated the Curie temperature of a ferromagnetic bilayer, demonstrating how interlayer coupling can influence properties at elevated temperatures, thereby emphasizing the significance of comprehending these dynamics in substance selection.
Consider External Fields: The impact of external fields on ferric materials cannot be understated. Powerful fields, especially those applied in opposition to the material’s inherent magnetism, can lead to significant demagnetization. For example, exposure to alternating electromagnetic fields in industrial environments can lead to temporary or even permanent loss of magnetism in ferrous components. This effect is particularly pronounced in environments where machinery and equipment are exposed to variable electromagnetic influences, necessitating careful consideration during procurement processes.
Examine Physical Damage: Physical impacts or structural alterations can profoundly disrupt the alignment of domains within iron, resulting in a loss of magnetism. Comprehending the mechanical robustness of electromagnetic components is essential for ensuring sustained performance in operational settings.
Review Environmental Factors: The question of whether is iron a magnetic element is relevant, as its attraction properties are also susceptible to environmental conditions. Factors like corrosion and oxidation can weaken the substance over time, further influencing its magnetic characteristics. Moreover, ferrimagnetic substances exhibit spontaneous magnetism below their Curie temperature, transitioning to paramagnetic above it. The interplay of these environmental factors, along with the behavior of ferrimagnetic substances, should be a key consideration in selection and long-term maintenance strategies.
Importance of Non-Sparking Tools in Explosive Environments: Besides the aforementioned factors, the choice of substances for environments with explosive potential is crucial. Non-Sparking Tools are essential alternatives in such applications, ensuring safety by minimizing the risk of ignition. These tools are commonly used in industries such as oil and gas, mining, and chemical processing, where the risk of sparks can lead to catastrophic events. Understanding the properties of these tools, especially in relation to magnetic materials and their behavior under various conditions, is crucial for procurement managers tasked with sourcing reliable equipment for hazardous environments.
Exploring Domadia’s Mica Tapes: Another critical consideration in high-temperature applications is the use of Domadia’s Mica Tapes. These tapes provide excellent electrical insulation at elevated temperatures, making them suitable for use in conjunction with Non-Sparking Tools. Their ability to withstand extreme heat without compromising performance is vital for ensuring safety and reliability in explosive environments. The integration of Mica Tapes with Non-Sparking Tools can enhance operational efficiency while maintaining compliance with safety standards.
Conclusion
Understanding the magnetic properties of iron is essential for making informed procurement decisions in various industrial applications. The exploration of ferromagnetism reveals how iron’s atomic structure, particularly its unpaired electrons, plays a crucial role in its magnetic behavior. This understanding not only highlights the importance of iron’s robust magnetic characteristics but also emphasizes the need to consider factors such as temperature and external influences that can affect its performance.
Comparing iron with other ferromagnetic metals like nickel and cobalt sheds light on their unique advantages and limitations. Iron’s cost-effectiveness and suitability for a wide range of applications, particularly in environments requiring non-sparking tools, make it a preferred choice for procurement managers. Recognizing the specific contexts where different magnetic materials excel allows for strategic material selection that aligns with project requirements and safety standards.
Moreover, the practical applications of iron’s magnetism—from electrical appliances to data storage—underscore its significance in modern technology. As industries continue to evolve, understanding the implications of iron’s magnetic properties will be vital for ensuring operational efficiency and safety. By leveraging this knowledge, procurement managers can navigate the complexities of material selection, ultimately contributing to safer and more effective industrial practices.




