Blogs

Understanding Types of Metallic Corrosion: A Complete Tutorial
Introduction
Metallic corrosion represents a pervasive challenge that can significantly undermine the integrity of materials across various industries. As environmental factors and chemical reactions contribute to the gradual degradation of metals, procurement managers must navigate the complexities of material selection to ensure safety and performance. This article delves into the fundamentals of metallic corrosion, exploring its types, influencing factors, and effective prevention strategies.
By understanding the intricacies of corrosion, including the role of innovative materials like Beryllium Copper and Copper Nickel alloys, procurement specialists can make informed decisions that enhance product reliability and operational efficiency. From the critical impacts on material performance to the strategic approaches for mitigation, this comprehensive overview equips professionals with the insights needed to combat corrosion effectively in their respective fields.
Introduction to Metallic Corrosion: Understanding the Basics
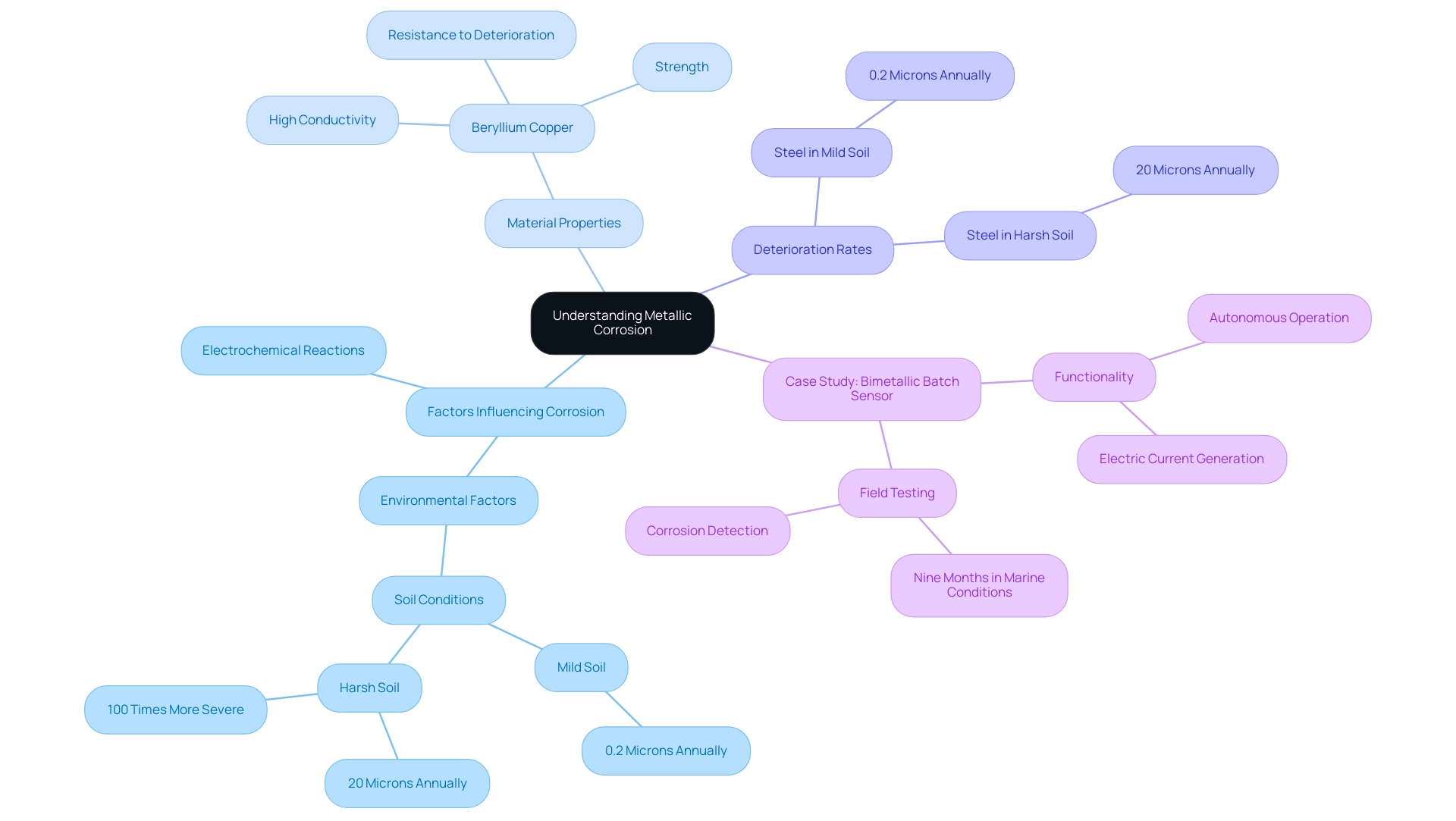
Metallic deterioration is the gradual degradation of metals caused by chemical reactions, primarily influenced by moisture and environmental factors. A comprehensive grasp of deterioration is crucial for preserving the integrity of substances utilized in production, as it can result in considerable product failures that endanger safety and efficiency.
For example, the deterioration rate of steel in soil varies from:
- 0.2 microns annually in mild soil
- 20 microns annually in harsh soil conditions
The deterioration rate in aggressive environments can be up to 100 times more severe than in favorable conditions. This highlights the need for vigilance in resource selection, particularly for procurement managers.
Domadia’s Beryllium Copper Plates exemplify excellence in strength, conductivity, and resistance to deterioration, making them an ideal choice for mitigating risks in various industrial applications. Our commitment to quality and customer satisfaction ensures that you are investing in reliable solutions that enhance performance and safety.
The main factors causing corrosion involve electrochemical reactions, which differ across various settings and can greatly influence the selection of substances during production processes. Beryllium Copper’s unique properties, such as its high conductivity and strength, allow it to withstand corrosive environments effectively, thereby prolonging the lifespan of components.
Procurement specialists must prioritize sourcing high-quality, corrosion-resistant materials like Beryllium Copper to ensure that products meet safety and performance standards.
A real-world example of deterioration evaluation can be observed in the case study of a bimetallic batch sensor created for monitoring degradation rates in steel reinforcement in concrete. This sensor functions autonomously, producing an electric current to signify deterioration intensity, and has demonstrated potential in identifying the shift from a passive state to degradation in environments with high chloride concentrations.
Exploring the Various Types of Metallic Corrosion
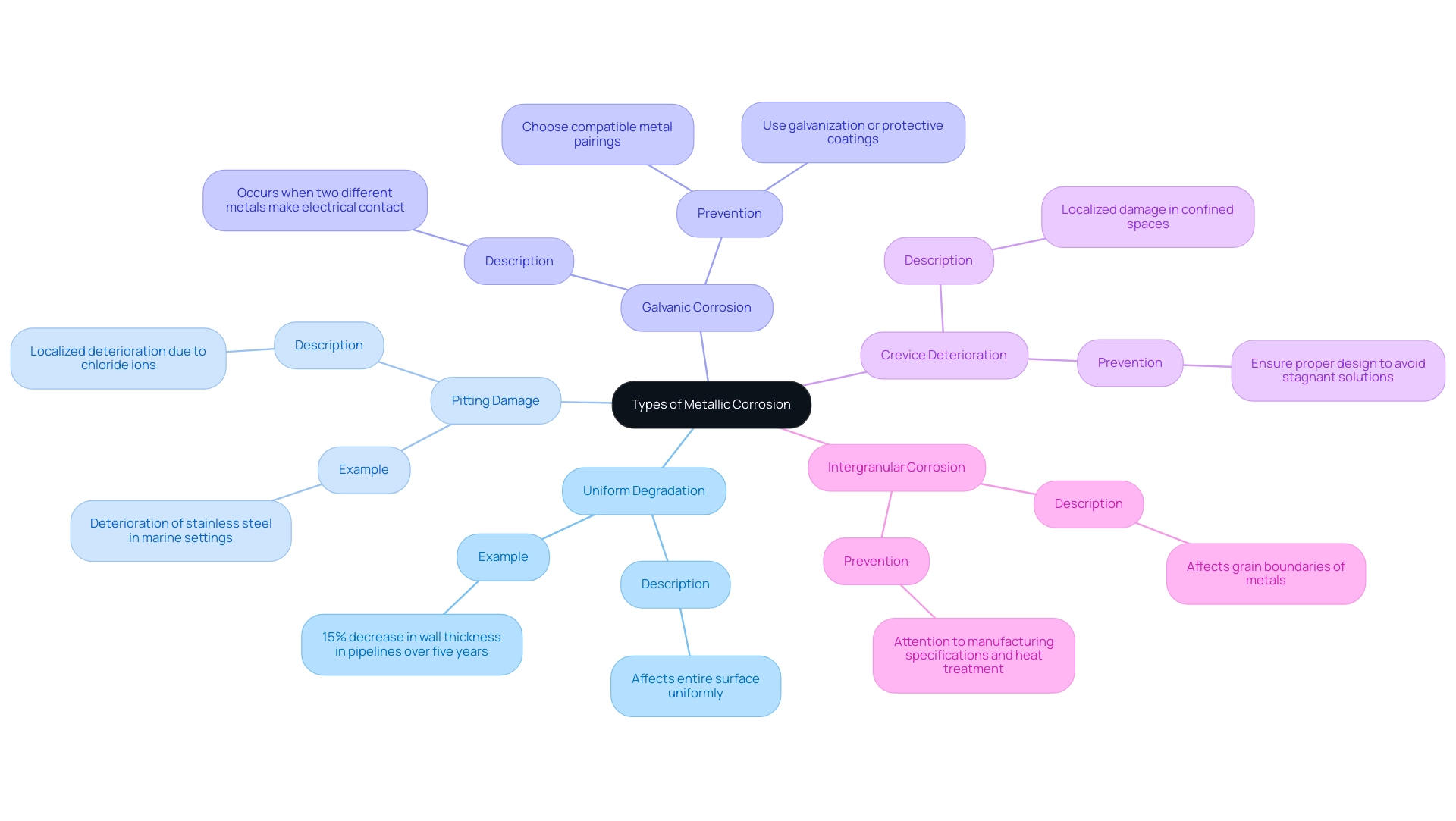
Metallic deterioration manifests in several distinct forms, each posing unique challenges to procurement managers tasked with material selection. Comprehending these types is essential for effective deterioration management:
Uniform Degradation: This type affects the entire surface of the metal uniformly, making it a predictable form of deterioration. It is often manageable through regular maintenance and monitoring. For example, in a study carried out in a chemical processing facility, uniform deterioration was noted on metal pipelines, resulting in a 15% decrease in wall thickness over five years, emphasizing the necessity for regular inspections.
Pitting Damage: Characterized by localized deterioration resulting in small holes or pits, pitting damage is frequently instigated by chloride ions. A case involves the deterioration of stainless steel in marine settings, where contact with saltwater resulted in pitting that weakened the structural integrity of a vessel, requiring expensive repairs. This form can lead to significant structural integrity issues if not promptly addressed.
Galvanic Corrosion: This happens when two different metals make electrical contact in a damaging setting, leading to increased deterioration of the less noble metal. To mitigate this, it is essential to choose compatible metal pairings and consider protective measures.
Crevice Deterioration: Found in confined spaces where stagnant solutions can accumulate, crevice deterioration leads to localized damage. Understanding the environment and ensuring proper design can help prevent this issue.
Intergranular Corrosion: This type affects the grain boundaries of metals and is often the result of inadequate heat treatment processes. Attention to manufacturing specifications and heat treatment protocols is essential to prevent this type of deterioration.
Each form of degradation necessitates strategic planning and careful selection of substances to mitigate risks. Recent statistics indicate that pitting deterioration incidents account for approximately 30% of all deterioration failures in industrial applications, emphasizing the importance of preventive measures. Additionally, innovative materials, such as microfine cements with average and maximum particle sizes of 4–6 and 15 μm, respectively, can enhance resistance to degradation while improving mechanical properties. As noted by Farrell,
Space has only allowed a very general introduction to metals to be given and readers are encouraged to expand their knowledge initially with the aid of the further reading list below.
This emphasizes the significance of ongoing education in the field, especially regarding the intricacies of metallic deterioration.
Factors Influencing Metallic Corrosion
Q2. What are the main benefits of utilizing copper nickel alloys?
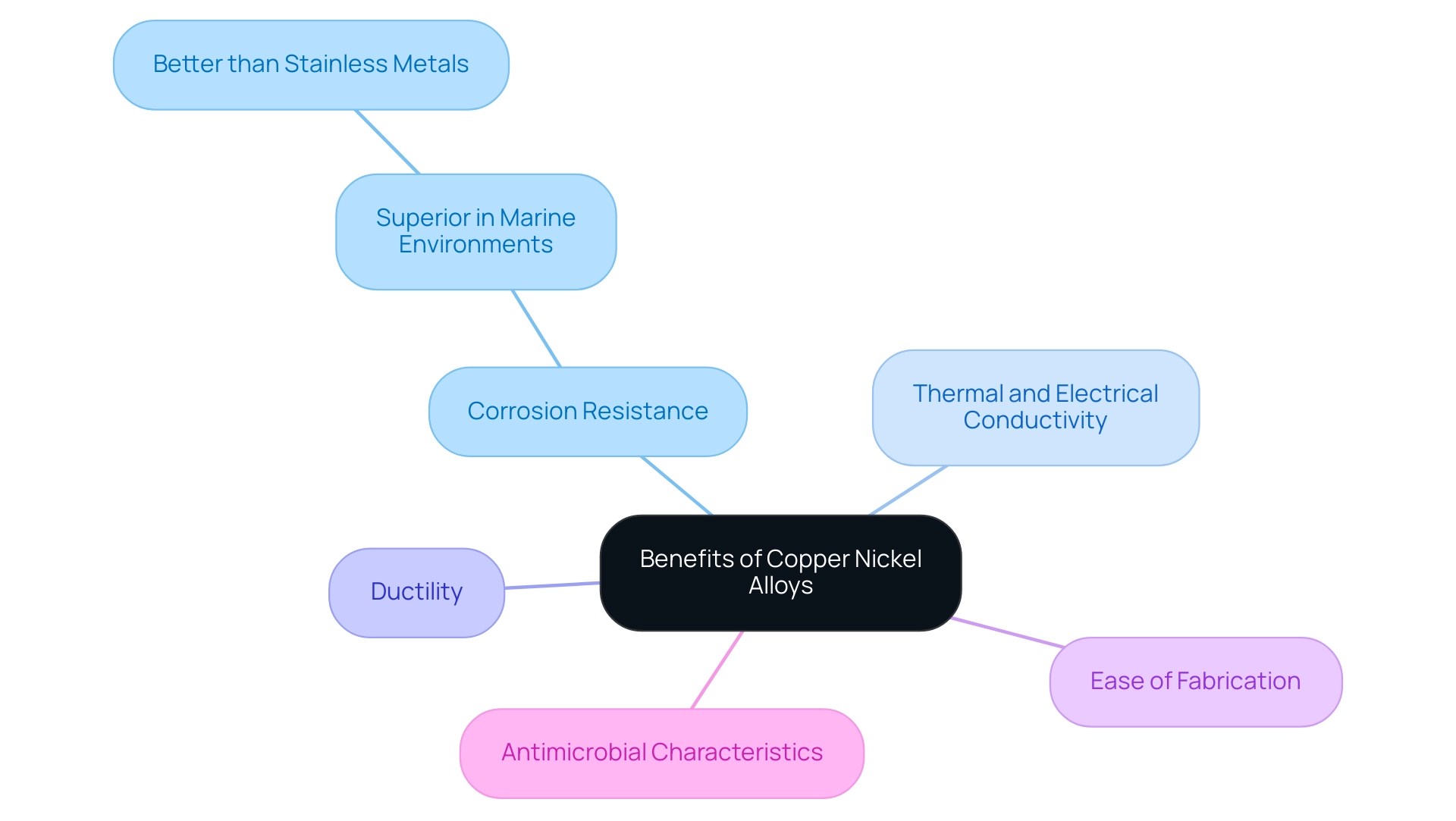
Copper nickel alloys provide various advantages, including:
– High resistance to deterioration in marine settings
– Excellent thermal and electrical conductivity
– Good ductility
– Ease of fabrication
– Antimicrobial characteristics, making them appropriate for applications where hygiene is crucial
How do copper nickel alloys measure up against other substances like stainless metals?
Copper nickel alloys excel over stainless metals in marine conditions because of their outstanding corrosion resistance, particularly in salty or brackish waters. They also have better thermal and electrical conductivity compared to stainless steel, making them ideal for heat exchangers and electrical applications. Their good ductility and ease of fabrication further enhance their usability, while their antimicrobial properties are essential for environments requiring high hygiene standards.
Prevention and Control Strategies for Metallic Corrosion
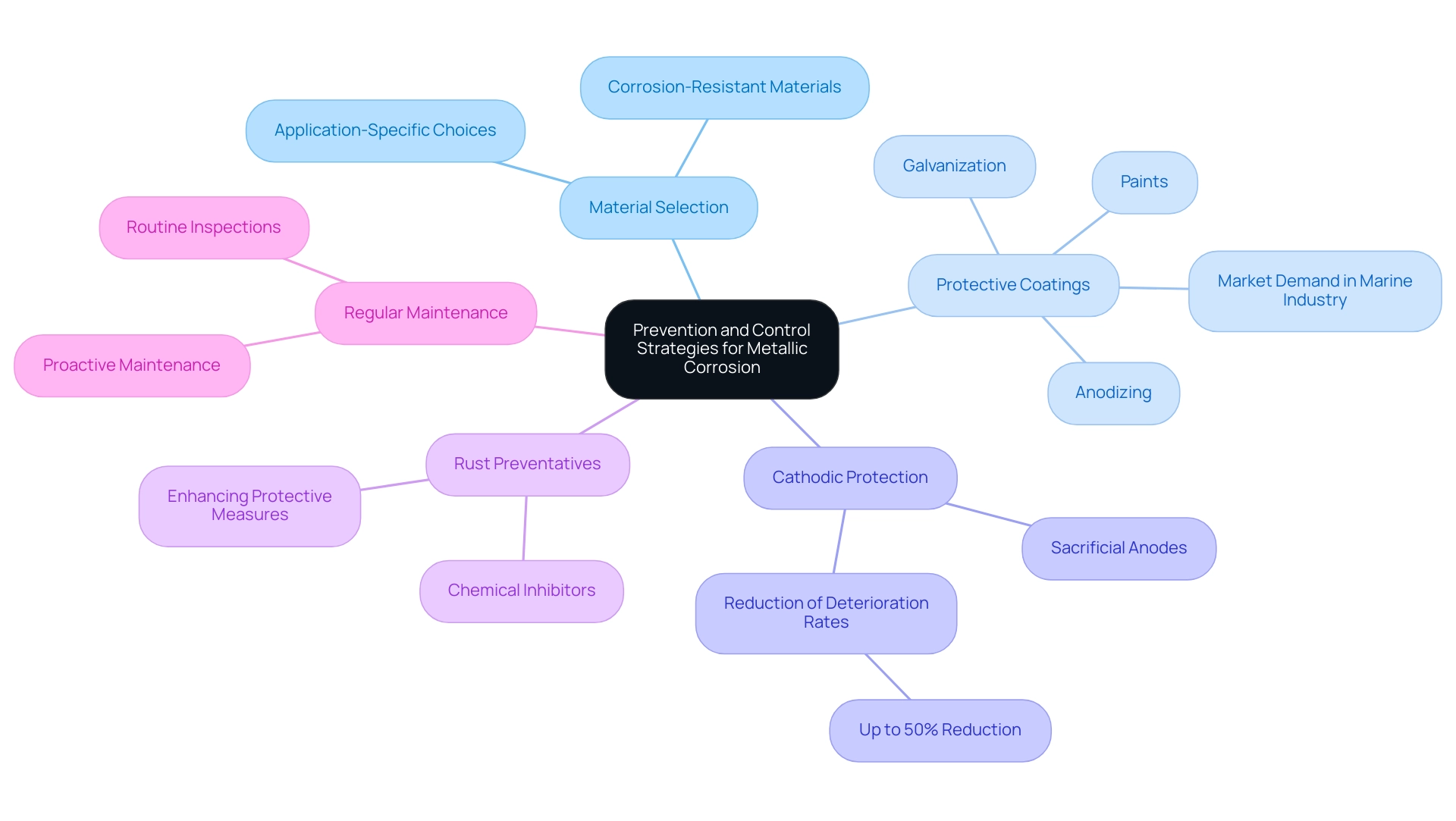
Effective prevention and control strategies for metallic corrosion are crucial for maintaining the integrity of substances across various applications. Key approaches include:
Material Selection: It is imperative to choose corrosion-resistant materials tailored to specific applications and environmental conditions. This strategic choice lays the foundation for long-term durability.
Protective Coatings: Implementing protective coatings—such as paints, galvanization, or anodizing—serves as a vital shield against corrosive elements. Recent market insights indicate that the demand for anti-corrosion coatings, particularly in the marine industry, is driving growth due to their efficacy in harsh environments. For instance, a case study from the marine industry highlights how the application of advanced protective coatings has significantly extended the lifespan of naval vessels operating in corrosive marine conditions.
Cathodic Protection: Utilizing sacrificial anodes is an effective method for safeguarding more valuable metals in galvanic deterioration scenarios. This strategy is especially notable in marine applications, where the risk of deterioration is heightened. According to recent statistics, the use of cathodic protection systems has been shown to reduce deterioration rates by up to 50%, making it a vital strategy for procurement managers to consider.
Rust Preventatives: The use of chemical inhibitors in settings vulnerable to rust can greatly reduce the pace of damage, providing an extra level of defense. Industry experts emphasize that selecting the right inhibitor can enhance the effectiveness of protective measures.
Regular Maintenance: Conducting routine inspections and maintenance is essential to identify and address deterioration issues early. By consistently monitoring the condition of resources, organizations can mitigate risks associated with corrosion-related failures. An expert in corrosion prevention notes, “Proactive maintenance is key to prolonging the life of critical assets exposed to corrosive environments.”
These strategies not only enhance the longevity of resources but also align with the overarching growth trends in the market, projected to expand at a CAGR of 5.3%. By adopting these measures, procurement managers can ensure the reliability and performance of their supplies, ultimately contributing to operational efficiency.
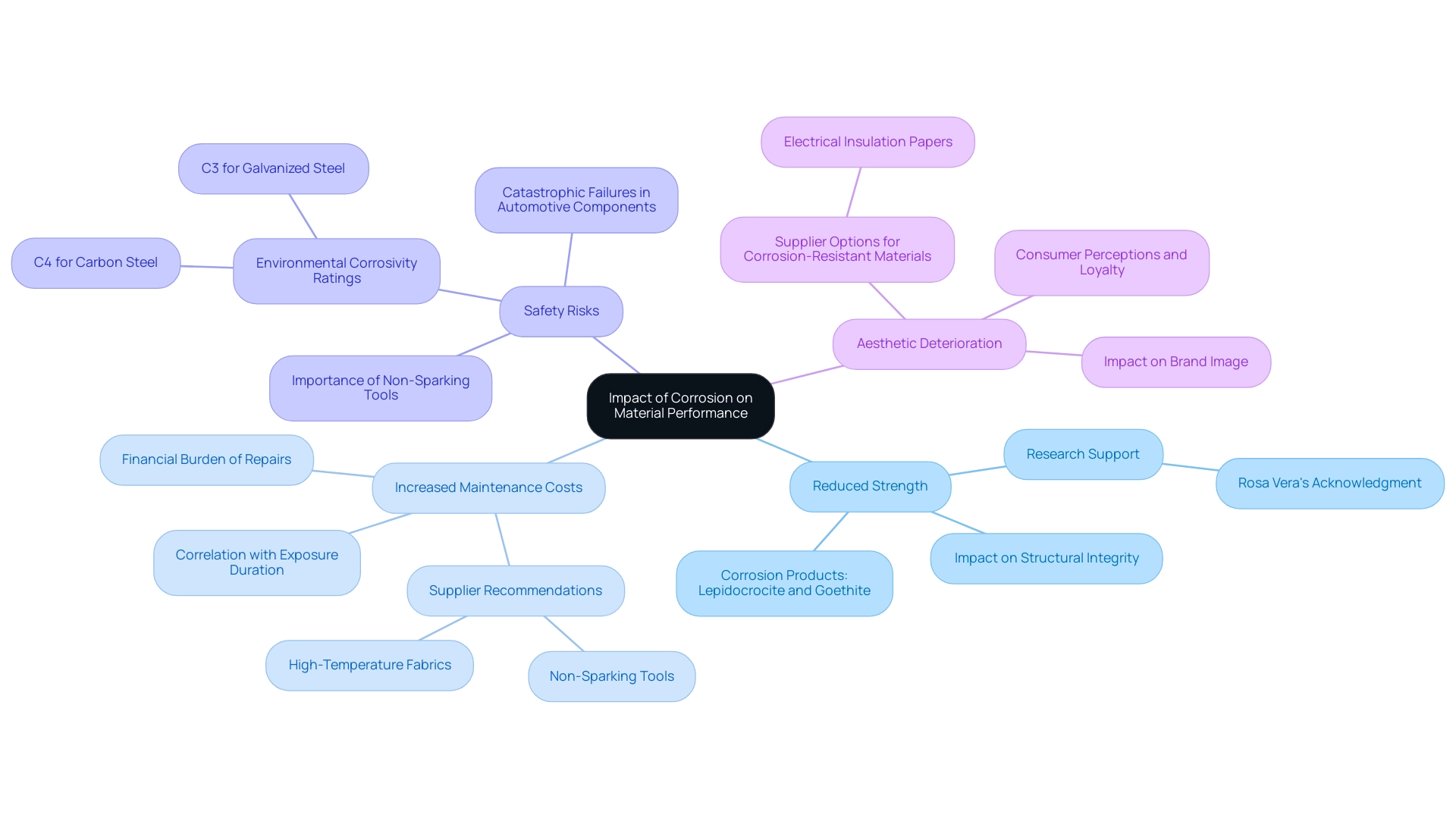
The Impact of Corrosion on Material Performance
Corrosion presents a range of detrimental effects on material performance that procurement managers must consider:
Reduced Strength: Corrosion diminishes the load-bearing capacity of metals, significantly impacting structural integrity. Recent studies have shown that substances created on carbon steel, such as Lepidocrocite and Goethite, weaken the structure’s strength over time, especially in tough conditions like Antarctica and Easter Island. As mentioned by Rosa Vera, “the authors gratefully acknowledge Chilean government support through funds from the Innova-CORFO Project No 09CN14-5879, Fondequip EQM 160091, and the Research Department from the Pontificia Universidad Católica de Valparaíso,” emphasizing the significance of research in understanding the effects of deterioration. Furthermore, in explosive settings, Non-Sparking Tools act as essential substitutes to reduce the risk of igniting combustible substances, highlighting the necessity for substances that withstand deterioration while ensuring safety. Notable suppliers of Non-Sparking Tools include [Supplier A] and [Supplier B], known for their durable and corrosion-resistant products.
Increased Maintenance Costs: The financial burden of maintaining corroded materials escalates as repairs or replacements become necessary. For instance, a study on the deterioration rate of carbon steel revealed a correlation between exposure duration and increased maintenance needs, indicating that initial deterioration rates can lead to higher long-term costs. Results indicated that deterioration rates diminished over time because of the protective barrier created by degradation products, emphasizing their function in reducing additional deterioration. Selecting Non-Sparking Tools made from corrosion-resistant materials can help reduce these maintenance costs in hazardous environments. Suppliers such as [Supplier C] and [Supplier D] offer high-temperature fabrics that complement these tools effectively.
Safety Risks: In critical applications, such as automotive components, deterioration can precipitate catastrophic failures. The environmental corrosivity categories evaluated revealed a concerning C4 rating for carbon steel and a C3 rating for galvanized steel, highlighting the potential for serious safety risks if deterioration is not effectively managed. Non-Sparking Tools, intended to function safely in explosive environments, must also be resistant to rust to guarantee ongoing safety and reliability.
Aesthetic Deterioration: Beyond functional implications, corrosion can tarnish the visual appeal of products, adversely affecting brand image and customer satisfaction. Procurement specialists must recognize that the appearance of supplies can influence consumer perceptions and loyalty. By choosing high-quality, corrosion-resistant substances and dependable suppliers, such as those providing Non-Sparking Tools and high-temperature options, procurement managers can improve product integrity and customer satisfaction. For instance, utilizing electrical insulation papers from [Supplier E] can further improve overall product resilience.
Understanding these impacts empowers procurement specialists to prioritize material quality and supplier reliability, ultimately enhancing product performance and customer satisfaction.
Conclusion
Metallic corrosion poses significant challenges across various industries, impacting material integrity and operational efficiency. By understanding the different types of corrosion—uniform, pitting, galvanic, crevice, and intergranular—procurement managers can make informed decisions that mitigate risks associated with these degradation processes. Each type of corrosion presents unique challenges that require tailored prevention strategies, highlighting the importance of vigilance in material selection.
Moreover, the exploration of effective prevention and control strategies emphasizes the critical role of high-quality materials, such as Beryllium Copper and Copper Nickel alloys. These innovative materials not only exhibit superior corrosion resistance but also enhance overall product reliability and safety. Implementing protective coatings, utilizing cathodic protection, and applying corrosion inhibitors are practical measures that can significantly reduce corrosion rates and prolong the lifespan of components in corrosive environments.
Ultimately, a strategic approach to managing metallic corrosion is essential for maintaining safety, performance, and cost-effectiveness. By prioritizing corrosion-resistant materials and employing proactive maintenance practices, procurement managers can safeguard their investments and ensure the longevity of their products. The insights shared in this article serve as a valuable resource for professionals aiming to navigate the complexities of corrosion and enhance their operational success.




