Blogs

Analyzing Nickel Corrosion: Mechanisms and Prevention Techniques
Introduction
Corrosion is a persistent challenge that significantly impacts the longevity and functionality of nickel alloys across various industries. This article delves into the intricate mechanisms of corrosion, exploring how environmental factors and material composition influence the degradation process. By examining different types of corrosion, including uniform, localized, galvanic, and stress corrosion cracking, it highlights the complexities involved in maintaining the integrity of nickel alloys.
Furthermore, it underscores the importance of strategic prevention techniques, such as protective coatings and alloying, to mitigate corrosion risks. Through insights from advanced research and collaborative efforts, the article emphasizes the critical role of understanding corrosion mechanisms in developing effective mitigation strategies, ultimately contributing to sustainable energy solutions and national security.
Understanding Corrosion Mechanisms
Corrosion is an inevitable phenomenon where metals degrade due to their interaction with environmental elements. For nickel-based mixtures, rust appears through various processes such as uniform degradation, localized deterioration, galvanic reaction, and stress-induced cracking. Each of these mechanisms is influenced by factors like temperature, pH levels, and the presence of corrosive agents. For instance, the inclusion of chlorides can speed up deterioration by compromising the passivity of the metal surface. Understanding these interfaces allows for precise component design, avoiding the pitfalls of overdesigning or underdesigning.
Localized degradation in nickel-based alloys can also arise from the presence of perchlorate, sulphate, and nitrate ions. To reduce these effects, various preventive actions can be utilized, including protective coatings like nickel plating and organic coatings, and combining nickel with other elements to improve its durability in particular settings. Sophisticated research techniques have also emphasized the limitations of conventional ‘cook-and-look’ methods, which only assess deterioration after it has taken place, resulting in conjectural theories regarding the deterioration process.
Recent advancements in rust resistance have been driven by collaborative research efforts, such as those supported by the Department of Energy’s Vehicle Technologies Office. These studies highlight the significance of comprehending deterioration processes to create effective mitigation strategies. This method not only enhances the durability and dependability of nickel materials but also aids in wider goals related to sustainable energy and national security.
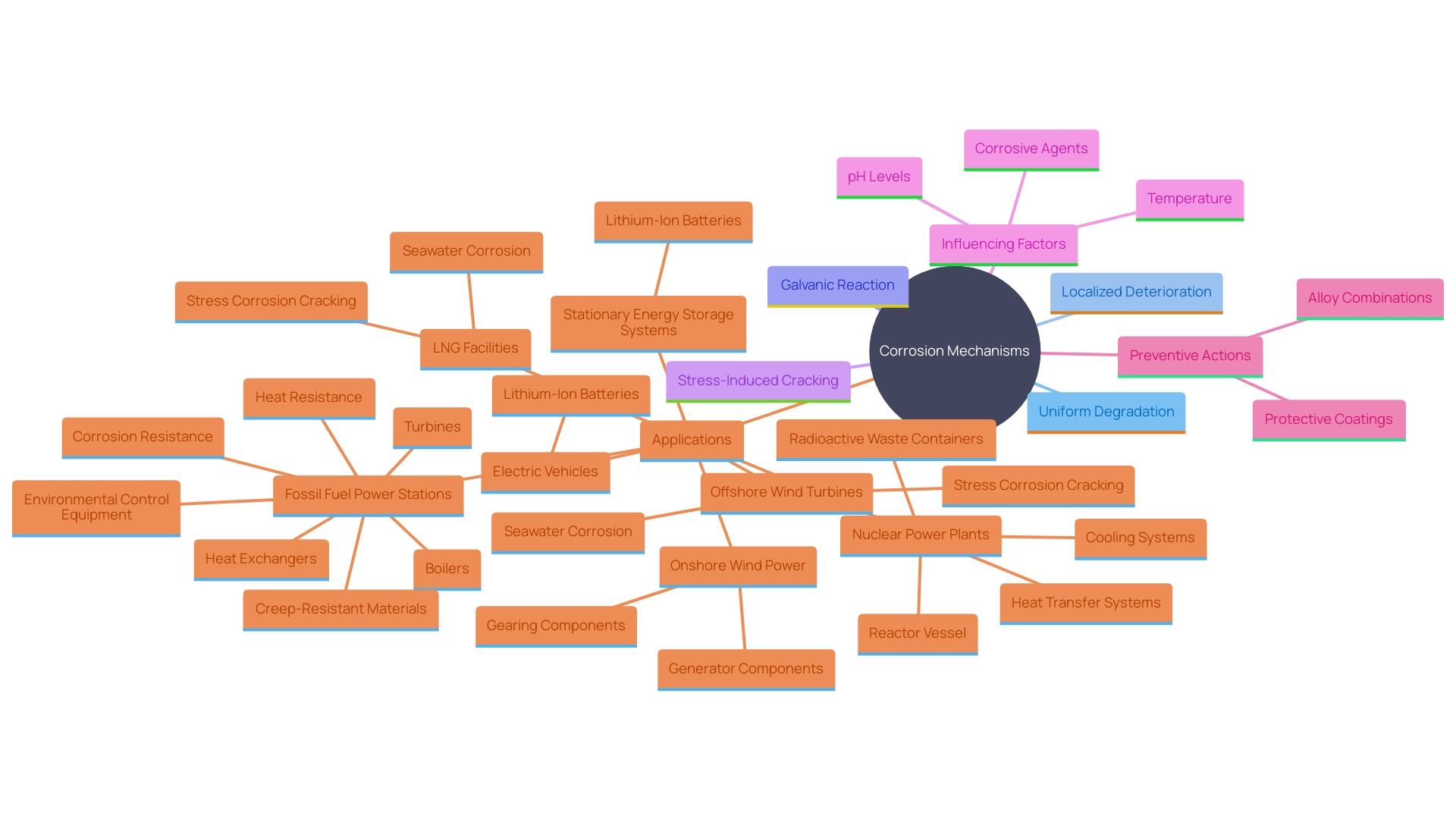
Types of Corrosion Affecting Nickel Alloys
Nickel alloys encounter different types of deterioration that can considerably affect their durability. Uniform deterioration leads to a steady, even material loss across the surface, gradually weakening the structure. Localized degradation, including pitting and crevice deterioration, results in intense, focused damage in specific areas, which can lead to severe structural failures. Galvanic deterioration occurs when nickel-based alloys make electrical contact with another metal in a harmful environment, speeding up the decline of the less noble metal. Stress-related cracking is especially alarming as it merges tensile stress and damaging elements, possibly resulting in abrupt and unforeseen breaks in components. Research has demonstrated that the existence of chlorides and other halides can worsen deterioration by dismantling the protective layers on metal surfaces. Efficient methods for preventing rust include using protective layers, such as plating with metals or organic coverings, and combining certain metals with elements like chromium and molybdenum to improve their durability in challenging conditions.
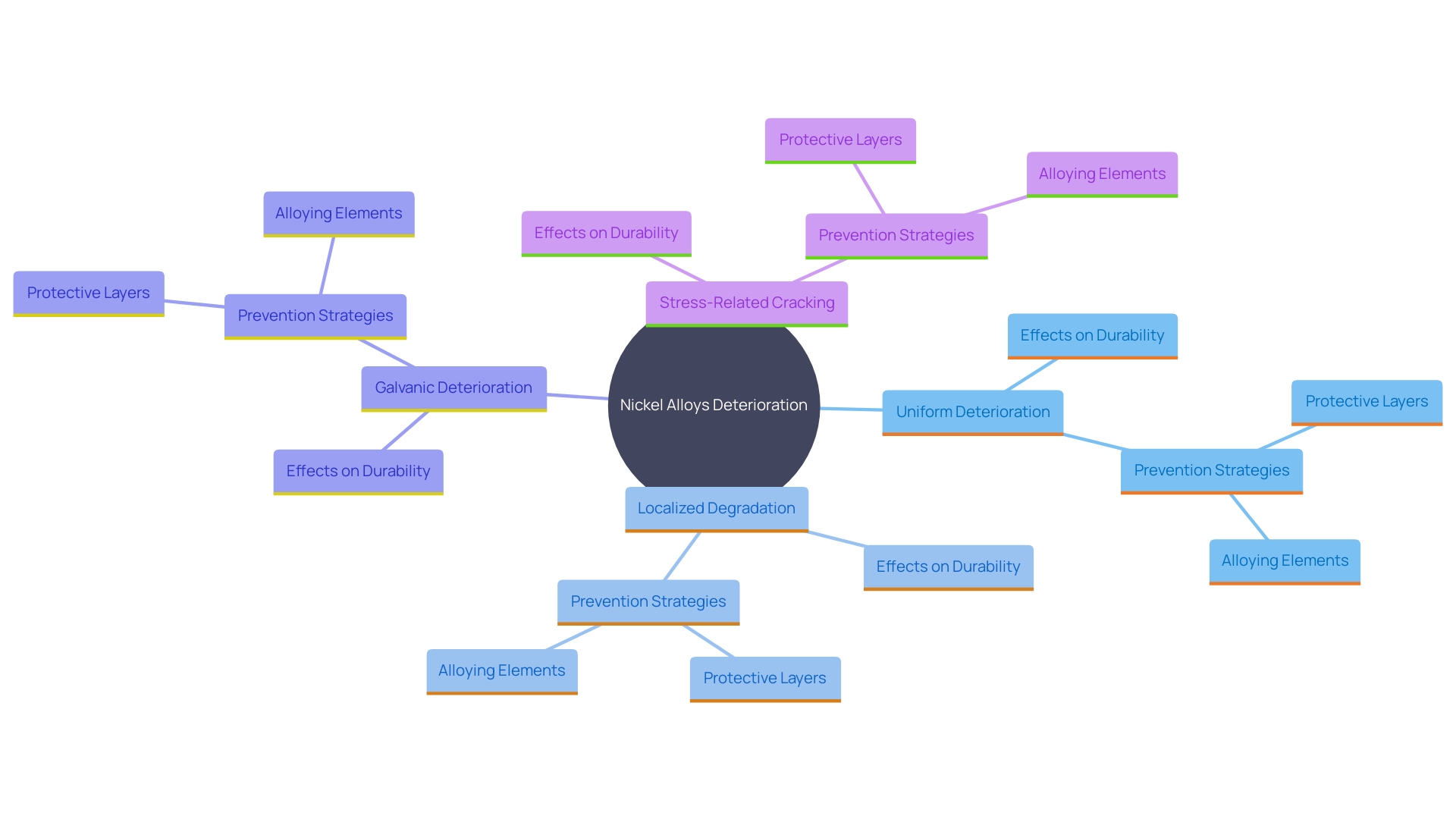
Factors Influencing Nickel Corrosion
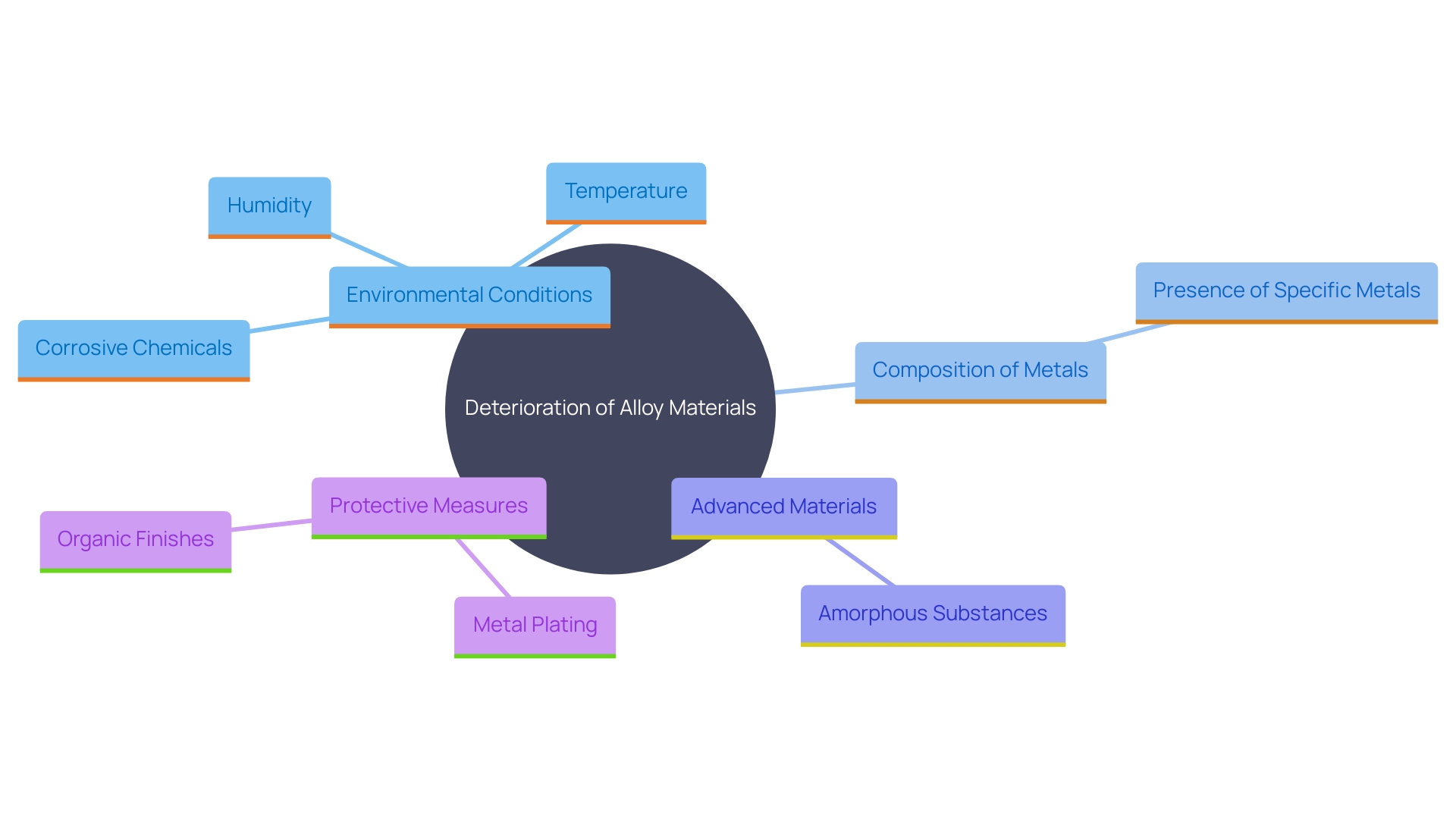
Various elements affect the speed and intensity of deterioration in alloy materials. Environmental conditions such as humidity, temperature, and exposure to corrosive chemicals play a critical role. For instance, the presence of chlorides and other halides can hasten the corrosion process by breaking down the passivity of the metal surface. The mixture of metals is another important aspect; increased presence of the specific metal usually improves durability. Certain advanced materials, such as amorphous substances, even demonstrate remarkable resistance to concentrated hydrochloric acids due to their unique atomic structure and uniform mixing of elements. Moreover, surface finish and microstructure are vital factors to take into account, as they influence how vulnerable a metal mixture is to deterioration. This is especially crucial in sectors such as nuclear energy, where specific metal alloys are utilized in both the heat transfer and cooling systems as well as within the reactor vessel. Applying protective layers, like metal plating and organic finishes, can also offer an additional barrier against rust. Comprehending these interfaces for deterioration enables more precise design, preventing excessive or insufficient designing of components, which is crucial for uses in extreme environments.
Prevention Techniques for Nickel Corrosion
Avoiding deterioration in nickel-based materials necessitates a tactical method that integrates design, choice of substances, and safeguarding techniques. Surface treatments such as passivation are essential by creating a protective oxide layer, improving durability against degradation. Additionally, applying coatings or barrier layers adds another line of defense against corrosive environments. Routine upkeep and observation are crucial to detect initial indications of deterioration, enabling prompt actions and prolonging the longevity of alloy components.
Materials that contain nickel are commonly utilized in different sectors because of their ability to withstand deterioration and longevity. For instance, LNG facilities and offshore wind turbines use a specific metal to combat seawater corrosion and stress corrosion cracking. In fossil fuel power plants, certain metal mixtures are selected for their heat resistance, enhancing the longevity of boilers and heat exchangers. In the same way, nuclear power facilities utilize metal alloys in both heat transfer and cooling systems, as well as in containers holding radioactive waste.
Comprehending the interfaces for deterioration is essential for precise design, preventing overdesigning or underdesigning components. This insight is supported by research funded by the Department of Energy’s Vehicle Technologies Office, highlighting the importance of nickel in sustainable energy and national security applications.
Furthermore, innovative studies have uncovered new information regarding water vapor’s interaction with metals at an atomic scale, with consequences for deterioration management. Techniques like environmental transmission electron microscopy (TEM) allow researchers to directly view molecular interactions, advancing our understanding of the corrosion process and improving preventative measures.
Conclusion
Corrosion presents a formidable challenge to the integrity and functionality of nickel alloys, impacting their application across a wide range of industries. The mechanisms of corrosion, including uniform, localized, galvanic, and stress corrosion cracking, reveal the complexity of this phenomenon and underscore the importance of understanding environmental influences and material composition. Key factors such as temperature, pH levels, and the presence of corrosive agents, particularly chlorides, play a critical role in accelerating the degradation process.
Effective prevention strategies are essential for mitigating corrosion risks associated with nickel alloys. Techniques such as protective coatings, alloying with other elements, and regular maintenance are vital for enhancing corrosion resistance and extending the lifespan of components. Research and advancements in understanding the interactions that drive corrosion have led to more precise design approaches, enabling engineers to avoid the pitfalls of overdesigning or underdesigning critical components.
In conclusion, the ongoing exploration of corrosion mechanisms and the implementation of strategic prevention techniques are crucial for maintaining the reliability of nickel alloys. This knowledge not only supports the durability of infrastructure in various industries but also contributes to broader objectives in sustainable energy solutions and national security. The commitment to advancing research and applying innovative solutions will ensure that nickel alloys continue to meet the demanding challenges presented by corrosive environments.




